Generic vs Brand Name Drugs: What the Label Really Tells You and Why They Work the Same
 Dec, 4 2025
Dec, 4 2025
Generic Drug Savings Calculator
How Much Do You Save?
Enter the monthly cost of your brand-name medication to see your potential savings with generics (FDA-approved generics cost 80-85% less).
Your Savings
Generic Price (per month):
Monthly Savings:
Annual Savings:
Value of Savings:
When you pick up a prescription, you might see two pills that look completely different-one is a big, colorful capsule with a fancy name, the other is a small, white tablet with no logo. You might wonder: is the cheaper one just as good? The answer is yes, and here’s why.
Same Active Ingredient, Different Look
Generic drugs and brand-name drugs contain the exact same active ingredient. That’s not a guess-it’s a legal requirement enforced by the U.S. Food and Drug Administration (FDA). Whether you’re taking omeprazole or Prilosec, atorvastatin or Lipitor, the molecule doing the work in your body is identical. The FDA requires that generic versions match the brand-name drug in strength, dosage form, route of administration, and intended use. If the active ingredient doesn’t match down to the last milligram, the generic won’t get approved.The differences you see? Those are all about appearance and marketing. Brand-name companies spend millions designing their pills to stand out-colors, shapes, logos. Generic manufacturers can’t copy that. U.S. trademark law forces them to make their pills look different. So a blue oval pill might become a white round one. That doesn’t mean it works any differently. It just means it’s not trying to look like the brand.
Label Differences: What You’re Actually Reading
Look closely at the label. The brand-name drug will say something like “Lipitor 20 mg.” The generic? It’ll say “Atorvastatin Calcium 20 mg.” That’s not a trick. The generic label uses the chemical name of the active ingredient, not the brand name. But here’s the key: everything else on the label-the warnings, side effects, dosage instructions, contraindications-is required by the FDA to be identical.The FDA’s 2021 guidance makes this crystal clear: generic drug labeling must mirror the brand’s in content and format. If the brand warns about muscle pain or liver enzyme changes, the generic must say the same thing. If the brand says “take with food,” the generic can’t say “take on an empty stomach.” This isn’t a loophole-it’s a rule. The FDA checks every single label before it hits the pharmacy shelf.
Therapeutic Equivalence: How the FDA Proves They Work the Same
The biggest concern people have is whether the generic gets absorbed the same way. After all, if your body doesn’t absorb it properly, it won’t work. That’s where bioequivalence testing comes in.Before a generic drug is approved, the manufacturer must run studies in 24 to 36 healthy volunteers. They measure two things: how fast the drug reaches peak concentration in your blood (Cmax) and how much of it gets absorbed over time (AUC). The generic must fall within 80% to 125% of the brand-name drug’s results. That’s not a wide range-it’s tight. In fact, the natural variation between two batches of the same brand-name drug can be wider than that.
Dr. Ameet Nagpal, a pharmacy expert at Northwestern Medicine, put it simply: “The 20% variability allowed in bioequivalence studies is actually tighter than the natural variation you see between different batches of the brand-name drug.” That means your body might react more differently to two pills from the same brand than it would to the brand versus the generic.
Cost: The Real Difference
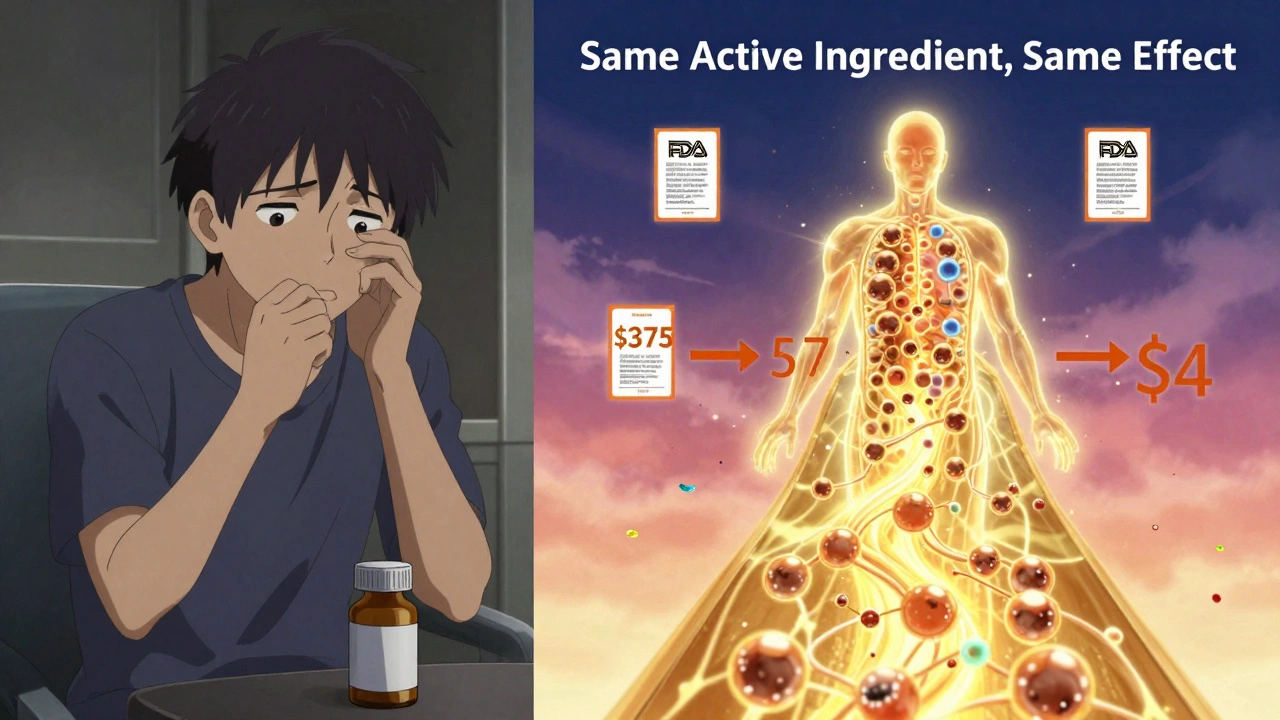
The only real difference between generic and brand-name drugs? Price. And it’s massive.Generic drugs cost, on average, 80% to 85% less. Atorvastatin (Lipitor) used to cost over $375 a month. Today, you can get the generic for under $4 at Walmart. Same molecule. Same effect. Same safety profile. Just a fraction of the cost.
That savings adds up fast. From 2007 to 2016, generic drugs saved the U.S. healthcare system $1.67 trillion. In 2023 alone, they saved $313 billion. The FDA says 90% of generics cost less than $10 a month. For people on fixed incomes, that’s the difference between taking their medicine and skipping doses.
When You Need to Be Extra Careful
Most of the time, switching from brand to generic is seamless. But there are exceptions. Drugs with a narrow therapeutic index (NTI) are trickier. These are medications where even a tiny change in blood level can cause serious problems-either the drug stops working or it becomes toxic.The FDA specifically calls out warfarin (for blood thinning), levothyroxine (for thyroid), and phenytoin (for seizures) as NTI drugs. For these, doctors often monitor blood levels more closely when switching between brands or even between different generic manufacturers. That’s not because generics are unsafe-it’s because the margin for error is razor-thin. One study in JAMA Internal Medicine followed 2 million patients on cardiovascular drugs and found no difference in outcomes between generics and brands. But for NTI drugs, caution is standard practice.
What Patients Actually Experience
Real-world feedback backs up the science. On Drugs.com, generic atorvastatin has a 6.6 out of 10 rating from over 1,800 reviews. Lipitor’s rating? 6.3. Not a big gap. Sixty-two percent of generic users report “moderate to significant” cost savings without any loss in effectiveness.But there’s one common complaint: confusion over appearance. A University of Michigan study found 12% of patients hesitated when they got a differently colored pill. One man on Reddit said he stopped taking his generic because he thought it was “the wrong medicine” after his pharmacist switched him to a new version. He didn’t realize the pill looked different because it came from a different manufacturer-not because it was inferior.
That’s why pharmacists are key. If you get a pill that looks unfamiliar, ask. Don’t assume something’s wrong. Most of the time, it’s just a different supplier. The FDA’s Orange Book tells you which generics are approved as therapeutically equivalent. If your pharmacist says it’s an “A-rated” generic, you’re good to go.
How Substitution Works in Practice
In 49 U.S. states, pharmacists can swap a brand-name drug for a generic without asking you-unless your doctor wrote “Dispense as Written” on the prescription. That’s the law. It’s designed to save money and keep people on their meds.The American Medical Association found that 94% of doctors feel comfortable prescribing generics. Most don’t even think twice. But if you’ve had a bad experience-say, you switched and felt off-talk to your doctor. Don’t assume the drug failed. It might be a different filler, or your body just needed time to adjust. For most people, though, the switch is invisible.
What’s Changing Now
The generic drug market is growing, not shrinking. In 2023, the FDA approved 79 complex generics-things like inhalers, injectables, and long-acting formulations. That’s up 22% from 2021. The first generic version of Ozempic (semaglutide) was approved in September 2023. That’s a big deal-it’s one of the most expensive drugs on the market.The FDA’s new Generic Drug Program Dashboard lets anyone track application status in real time. And the agency’s 2024-2028 plan aims to cut review times for complex generics by 20%. Meanwhile, over $268 billion in brand-name drug sales are set to lose patent protection by 2028. More generics are coming.
Bottom Line: Trust the Science, Not the Label
Generic drugs are not second-rate. They’re not cheaper because they’re weaker. They’re cheaper because they don’t need to recoup billions in marketing and R&D costs. The FDA holds them to the same standards. The active ingredient is identical. The label is identical. The safety profile is identical. The only thing that changes is the price.If you’re worried about switching, talk to your pharmacist. Ask if the generic is FDA-approved and A-rated. If you’re on a high-risk medication like warfarin or levothyroxine, ask your doctor to check your blood levels after the switch. But for 99% of prescriptions-antibiotics, blood pressure pills, cholesterol meds, antidepressants-there’s no reason not to choose the generic.
You’re not sacrificing quality. You’re gaining access.
Are generic drugs really as effective as brand-name drugs?
Yes. The FDA requires generic drugs to contain the exact same active ingredient, in the same strength, and delivered the same way as the brand-name version. They must also prove they’re absorbed into the body at the same rate and to the same extent through bioequivalence testing. Studies involving millions of patients show no meaningful difference in effectiveness or safety between generics and brand-name drugs.
Why do generic pills look different from brand-name ones?
U.S. trademark laws prevent generic manufacturers from copying the shape, color, or markings of brand-name pills. That’s why a blue Lipitor capsule might become a white atorvastatin tablet. These visual differences are purely for legal reasons-they don’t affect how the drug works. The active ingredient and its performance in your body remain unchanged.
Can I trust a generic drug if it’s much cheaper?
Absolutely. The lower price comes from not having to repeat expensive clinical trials or spend millions on advertising. Generic manufacturers only need to prove bioequivalence, which is far less costly. The FDA inspects manufacturing facilities for generics just as strictly as for brand-name drugs. There’s no difference in quality control.
Are there any drugs where I should avoid generics?
For most drugs, no. But for narrow therapeutic index (NTI) drugs-like warfarin, levothyroxine, and phenytoin-small changes in blood levels can matter. If you’re on one of these, your doctor may monitor your levels more closely after switching. But even then, generics are safe and effective; they just require more careful management.
How do I know if my generic is FDA-approved?
Check the FDA’s Orange Book, which lists all approved generic drugs and their therapeutic equivalence ratings. Look for an “A” rating, which means the generic is approved as equivalent to the brand. Your pharmacist can also confirm this. If it’s on the market and sold legally in the U.S., it’s been reviewed and approved by the FDA.
Why do I sometimes feel different when switching between generic brands?
Different generic manufacturers may use slightly different inactive ingredients-fillers, dyes, or binders. While these don’t affect the active drug, a small number of people report sensitivity to certain fillers, which can cause minor side effects like stomach upset. If you notice a change after switching generics, talk to your pharmacist. They can check if you’re getting a different manufacturer and possibly switch you back.


zac grant
December 4, 2025 AT 18:59Let’s be real-the FDA’s bioequivalence standards are insane. 80-125% AUC and Cmax? That’s tighter than the natural variation between two batches of the same brand-name drug. Most people don’t realize that. You’re not getting a ‘lite’ version-you’re getting the exact same pharmacokinetics, just without the marketing budget.
And the label? Identical warnings, same dosage instructions. The only thing different is the color. I’ve switched my patients to generics for a decade. Zero clinical difference. Just way more money in their pockets.
Also, the Orange Book is your best friend. If it’s A-rated, you’re golden. No need to overthink it.
Alex Piddington
December 4, 2025 AT 19:22It is important to note that generic medications are subject to the same rigorous manufacturing standards as their branded counterparts. The U.S. Food and Drug Administration conducts inspections of all facilities, regardless of whether they produce brand-name or generic products. Quality control is not compromised.
Furthermore, the cost savings are not merely anecdotal; they are quantifiable and have resulted in billions of dollars in savings for the healthcare system. This is not just a personal benefit-it is a public health imperative.
Libby Rees
December 6, 2025 AT 12:17People get scared when the pill looks different. I get it. But it’s just the dye. Same active ingredient. Same effect. If you’re worried, ask your pharmacist. They know the Orange Book better than most doctors.
Dematteo Lasonya
December 7, 2025 AT 11:39I switched my blood pressure med to generic last year and didn’t notice a thing. My BP stayed stable. My wallet thanked me. Just wanted to say thanks to whoever wrote this-it’s nice to see the science laid out so clearly.
Also, my pharmacist gave me a little card that explained the difference. Best thing ever.
Gillian Watson
December 9, 2025 AT 06:46Same active ingredient same results same FDA approval just cheaper. Why is this even a debate
Also love how the UK does this. Everyone takes generics. No one bats an eye.
Jordan Wall
December 9, 2025 AT 12:41LOL at the ‘FDA says it’s safe’ narrative. 🤡 You really think the same people who approved Vioxx and OxyContin are your healthcare heroes? 🤔
And don’t even get me started on the ‘bioequivalence’ loophole. 80-125%? That’s a 45% swing, bro. That’s not precision-that’s a casino.
Also, why do generics always have that weird chalky taste? Coincidence? I think not. 🧪
Gareth Storer
December 10, 2025 AT 15:18So you’re telling me the $4 pill my pharmacist slipped me is the same as the $375 one? Cool. I’ll believe it when my insurance stops forcing me to take it.
Also, I tried switching my antidepressant and felt like a zombie for two weeks. So no, not all generics are created equal. Some of us aren’t lab rats.
Pavan Kankala
December 12, 2025 AT 09:22Big Pharma doesn’t want you to know this, but generics are just recycled waste from Chinese factories. The FDA is bought off. They don’t test anything. The real drug is in the brand. The rest is placebo with a barcode.
My cousin in India got a fake generic that made his liver explode. That’s the truth they don’t show you. Wake up.
And why do all generics have the same white color? Because they’re all made in the same factory. Controlled by the same cartel. You’re being manipulated.
Martyn Stuart
December 14, 2025 AT 09:14It’s worth noting, however, that while the vast majority of generic drugs are perfectly safe and effective, there are occasional, rare instances where differences in inactive ingredients-such as fillers, binders, or dyes-can trigger minor adverse reactions in sensitive individuals. This is not a flaw in the generic system, but rather a reminder that human biology is complex.
That’s why, if you experience any change in how you feel after switching, it’s entirely reasonable-and advisable-to consult your pharmacist or prescriber. They can verify the manufacturer, check the Orange Book, and even request a specific brand if needed. Your health is worth the extra step.
val kendra
December 15, 2025 AT 21:00My grandma takes 7 meds. 6 are generic. She’s 82 and still hikes every weekend. She doesn’t know what ‘bioequivalence’ means, but she knows her pills work and she’s not broke.
Stop overcomplicating it. The science is solid. The savings are real. If you’re not on warfarin or levothyroxine, just take the damn generic and save your cash for pizza.